

How to get a test ?
Medicare part B now covers this test for qualifying patients. We will mail a self-saliva test kit upon checking and verifying your eligibility.
Program Eligibility : Symptomatic individual 65 years or older with clinical diagnosis or suspicions of one or more of the following conditions:
*** Registering will allow us to send you information and updates via text message and email. You can unsubscribe anytime by replying STOP or UNSUBSCRIBE.

How to get a test ?
Medicare part B now covers this test for qualifying patients. We will mail a self-saliva test kit upon checking and verifying your eligibility.
Program Eligibility : Symptomatic individual 65 years or older with clinical diagnosis or suspicions of one or more of the following conditions:
*** Registering will allow us to send you information and updates via text message and email. You can unsubscribe anytime by replying STOP or UNSUBSCRIBE.
Majors (Diagnosed Disease)
- EARLY ONSET OR SEVERE AUTOIMMUNITY DISORDERS
- Type 1 Diabetes
- Rheumatoid Arthritis
- Psoriatic Arthritis
- Multiple Sclerosis
- Systemic Lupus Erythematosus
- Bone Marrow Failure Syndromes
- Nezelof’s syndrome
- DiGeorge Syndrome (DELAYED DEVELOPMENT)
- Chronic inflammatory demyelinating polyneuropathy
- FREQUENT AND RECURRENT INFECTIONS
- Pneumonia
- Chronic bronchitis
- Chronic Sinusitis UNSP
- Chronic Ear Infections
- Meningitis
- Skin infections
- INFLAMMATION AND INFECTION OF INTERNAL ORGANS
Cause of inflammation and infectious of internal organs the most common reasons for chronicinflammation include – Autoimmune disorders, such as lupus, where your body attacks healthy tissue. Exposure to toxins, like pollution or industrial chemicals. Untreated acute inflammation, such as from an infection or injury.
- Crohn’s Disease
- BLOOD DISORDERS
- Low platelet counts (Thrombocytopenia, unspecified)
- Anemia – D64.9
- Hemophilia – All bleeding disorders
- All LYMPHOMAS
- Hyperthyroidism and Hypothyroidism
- Early or very early onset other inflammatory bowel disease
- Ulcerative colitis
- Guillain - Barre Syndrome
- Neurologic abnormalities
- Alzheimer’s
- Dementia
- Parkinson’s Disease
Minors (conditions/symptoms of a disease)
Other clinical signs may be present that warrant evaluation for the presence of primary immunodeficiency
- Multiple, unusual, or severe allergies
- Severe eczema/atopic dermatitis
- Ectodermal dysplasia
- Abnormal hair
- Enteropathy
- Autoimmune Lymphoproliferative disease
- Hemophagocytic lymph histiocytosis
- Skeletal Dysplasia – Chondrodysplasia
- Digestive Problems
- Cramping
- Loss of appetite
- Nausea
- Diarrhea
Why is it important to establish a molecular diagnosis in primary immunodeficiency disorders?
This information allows healthcare providers to better inform affected individuals and families about prognosis and optimal surveillance strategies, and may also guide therapy. For example, patients with severe combined immunodeficiency might have similar phenotypes even though they have variants in different genes. Knowing which gene is affected helps determine whether the best treatment option might be enzyme replacement therapy, gene therapy, or a stem cell transplant.
In addition, once the molecular diagnosis is established, healthcare providers can better define the risk of recurrence and decide whether to screen unaffected family members for carrier status, to identify a suitable stem cell donor, or for other purposes.

How has the diagnostic approach to immunodeficiency disorders changed in recent years?
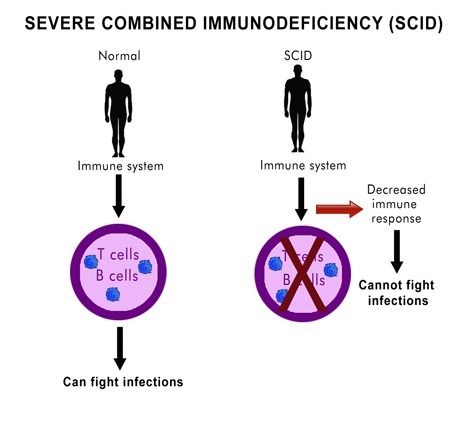
Historically, when a patient presented with a clinical history suspicious for a primary immunodeficiency, the diagnostic process began with a basic immunological evaluation and subsequent functional studies to identify the specific immunological defect. Then, after narrowing the differential diagnosis or making a clinical diagnosis, labs might have performed genetic studies, if available, to establish a molecular diagnosis.
However, the clinical and genetic heterogeneity of some primary immunodeficiency disorders can make this approach challenging. The phenotypic spectrum is quite broad for some disorders, resulting in the same molecular defect presenting quite differently among individuals. In addition, given the rare nature of some of these disorders, a patient might present with a phenotype that isn’t yet fully characterized. These scenarios can make it difficult to select for sequencing the gene(s) most likely to cause disease. If a lab sequences multiple individual genes separately, testing can also be cost prohibitive when the immunologic work-up does not narrow the diagnosis to one or a few genes.
With advances in technology, genetic testing has drastically decreased in cost and become more widely available. In particular, the introduction of massively parallel or next-generation sequencing (NGS) has given labs an economically feasible way to test many genes simultaneously, which contrasts with traditional Sanger sequencing approaches that typically test only one or a few genes at a time. These advances, now available in clinical settings, have led to an alternate “genotype-first” approach to the diagnosis of immunodeficiency disorders. Additionally, widespread genetic testing has resulted in a rapid increase in the identification of monogenic immunodeficiency disorders.
Which ngs test approach is most appropriate for immunodeficiency disorders—a targeted panel or exome sequencing?
There are pros and cons to both approaches. Targeted panels test an expertly curated list of genes associated with the disease of interest and can include variants in the exons and known variants located in non-coding regions. These panels may also involve complementary methods for genes difficult to test by NGS. However, it’s challenging for labs to keep targeted panels updated as additional genes become associated with disease. Exome sequencing, which tends to be more expensive and have a longer turnaround time, might not include important non-coding variants or key genes that are not amenable to NGS. However, exome sequencing allows for the discovery of additional disease-associated genes and might be more suitable for individuals with a nonspecific phenotype who might otherwise require multiple targeted panels.

Are there limitations to genetic diagnosis?
Despite advances in testing, labs still aren’t able to reach a genetic diagnosis for all patients. This can be due to a gene that has not yet been associated with disease, oligogenic or polygenic inheritance, epigenetics, or other variables. Healthcare providers should therefore continue to integrate genetic information with clinical history and functional studies when making a diagnosis. This requires close collaboration between clinicians and laboratory professionals.
